
7 Myths About Sugar Everybody Believes
What if I told you everything you knew about sugar is wrong? Like not just a little wrong, but so wrong, you might even get a brain scan after realizing all the lies you believed about sugar.
The model of muscle growth is misunderstood by the average person as is, but many professionals don’t come close either. Regardless whether you’re a coach or simply an enthusiast, let me teach you about what does and doesn’t drive muscle growth.
And as a heads up, this will be one of my more textbooky sounding articles because topics like this requires this level of nerdiness. So I apologize in advance if I don’t crack as many jokes as I usually do.
But seriously, stick around till the end. You’ll be smarter than most so called bodybuilding coaches.
Muscle anatomy starts with a muscle cell which is also a muscle fiber. Most people don’t know that those 2 are the same things. Each muscle cell or fiber contains some glycogen and fat, a bunch of myofibrils (which I’ll talk about in a second) and mitochondrion (plural word for mitochondria).
A muscle cell is actually the long cylinder fiber looking shape you probably thought a muscle fiber was. Again, they’re the same thing. Muscle cells are not your traditional looking cell, but rather strand like in shape. Each muscle cell/fiber contains lots of myofibrils which are like a bunch of little fibers.
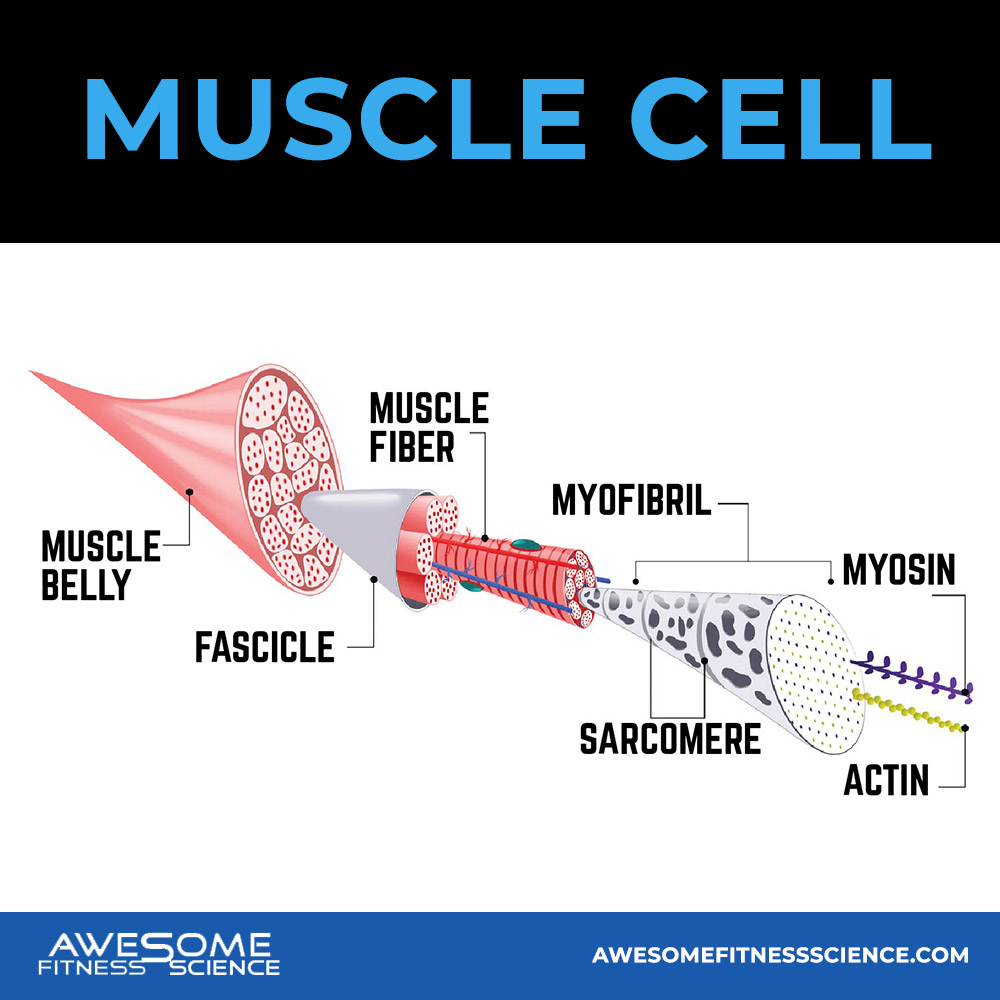
Across each myofibril are sarcomeres which are little sections of contractile units all around a myofibril.
These sarcomeres are made up of actin and myosin which are protein filaments. When muscles contract, actin and myosin form cross bridges to produce force (1). Actin and myosin are essentially having sex. The more overlap they have, the more force myofibrils produce and thus, the more force produced from the individual muscle fiber.
Furthermore, titin is a third protein filament, but is only active when muscles lengthen and not shorten (2).
Each muscle fiber are grouped together into hundreds of teams within a muscle. These teams are called motor units and they differ in size, muscle fiber type, and the the number of muscle fibers within them.
When your brain wants your muscles to produce force, it sends an electrical signal to activate these teams of muscle fibers also called motor units as I mentioned (3).
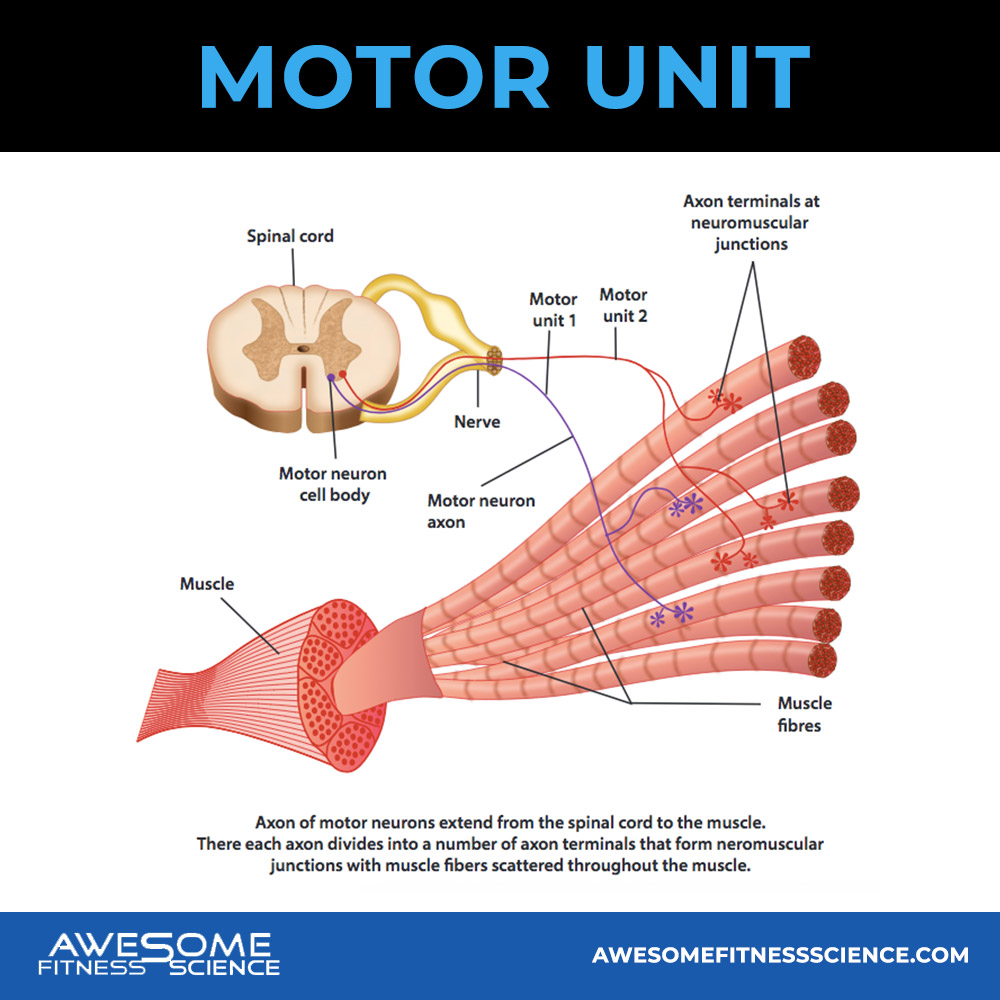
Motor units can be divided into 2 types, small and big. Smaller motor units generally have more type 1 fibers which produce less force over longer durations, but also contain fewer total muscle fibers per unit.
Bigger motor units generally have more type 2 fibers which produce more force, but fatigue faster while containing more muscle fibers per unit. Type 2 fibers also have more potential for growth (4).
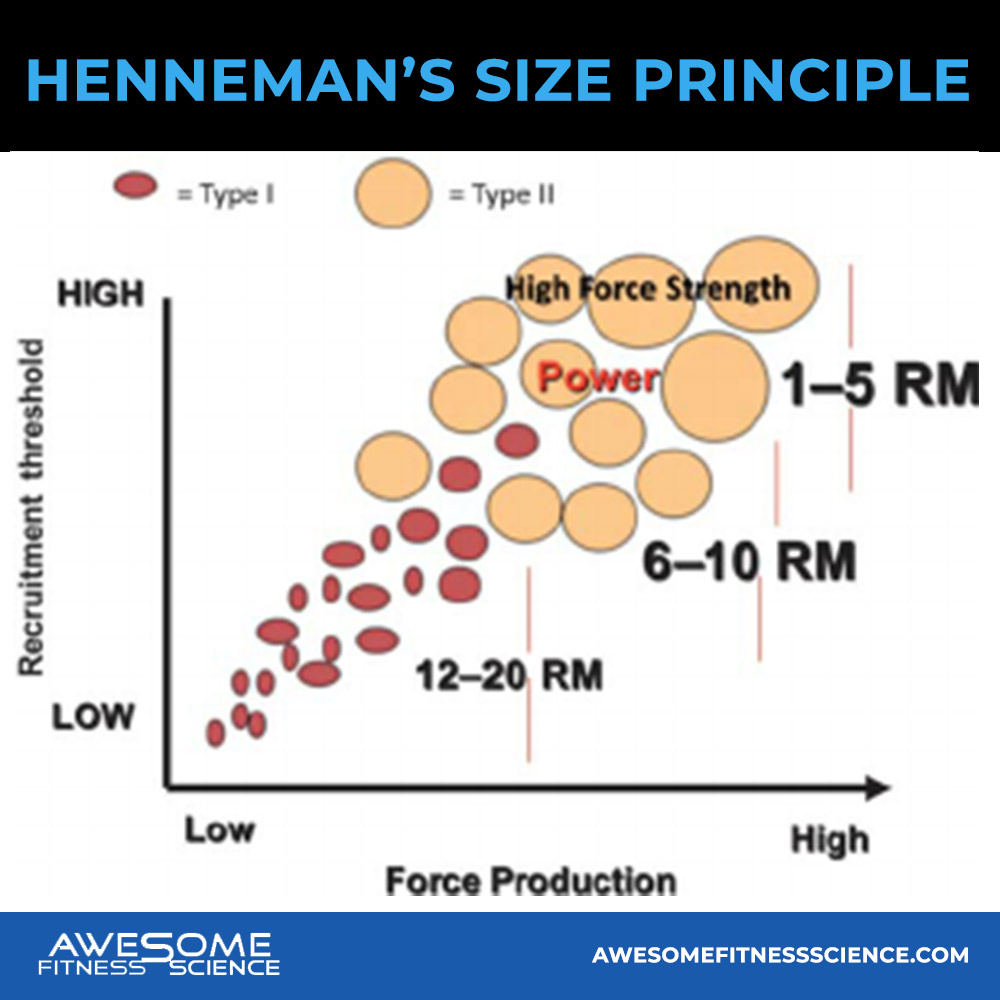
When your brain activates motor units and recruits muscle fibers, it does so in a specific order. It initially recruits small motor units also called low threshold motor units because the threshold for turning them on is well, low.
Conversely, bigger motor units are also called high threshold motor units because the threshold for them to activate are higher. They don’t turn on unless they are needed like when smaller motor units start to fatigue or the resistance demand is too high for the smaller motor units to tackle alone. This is called the Henneman’s size principle (8,9,10)
Additionally, full motor unit recruitment occurs when you have to produce 75-85% of maximal force (5,6). Beyond that, to produce further force, your body must increase force production by increasing the rate of firing frequencies (how fast it sends those activation signals) (7,12).
So now you know how muscles produce force. However, total muscle force and individual muscle fiber force are 2 important distinctions for understanding muscle growth.
Let’s say I had a pie, ready to smack it as hard as I can into the face of some clown. My brain would intend to move my arm as quickly as I can, so the external force required is strong enough for the most epic pie to face explosion ever.
Because external force is high, full motor unit recruitment will occur which is good because lots of muscle fibers are activated. However, activation alone doesn’t cause muscle growth. While, the external force was high, each muscle fiber was required to contract quickly due to the fast nature of pie slapping.
When muscles contract quickly, their individual muscle force is low and thus, mechanical tension is not sufficient to stimulate muscle growth. To impose a robust amount of mechanical tension, your muscles must be activated and the individual fibers need to contract slowly to produce high levels of force.
This is called the force-velocity relationship (13,14,15). The slower the contraction, the more force is produced from the individual fiber.
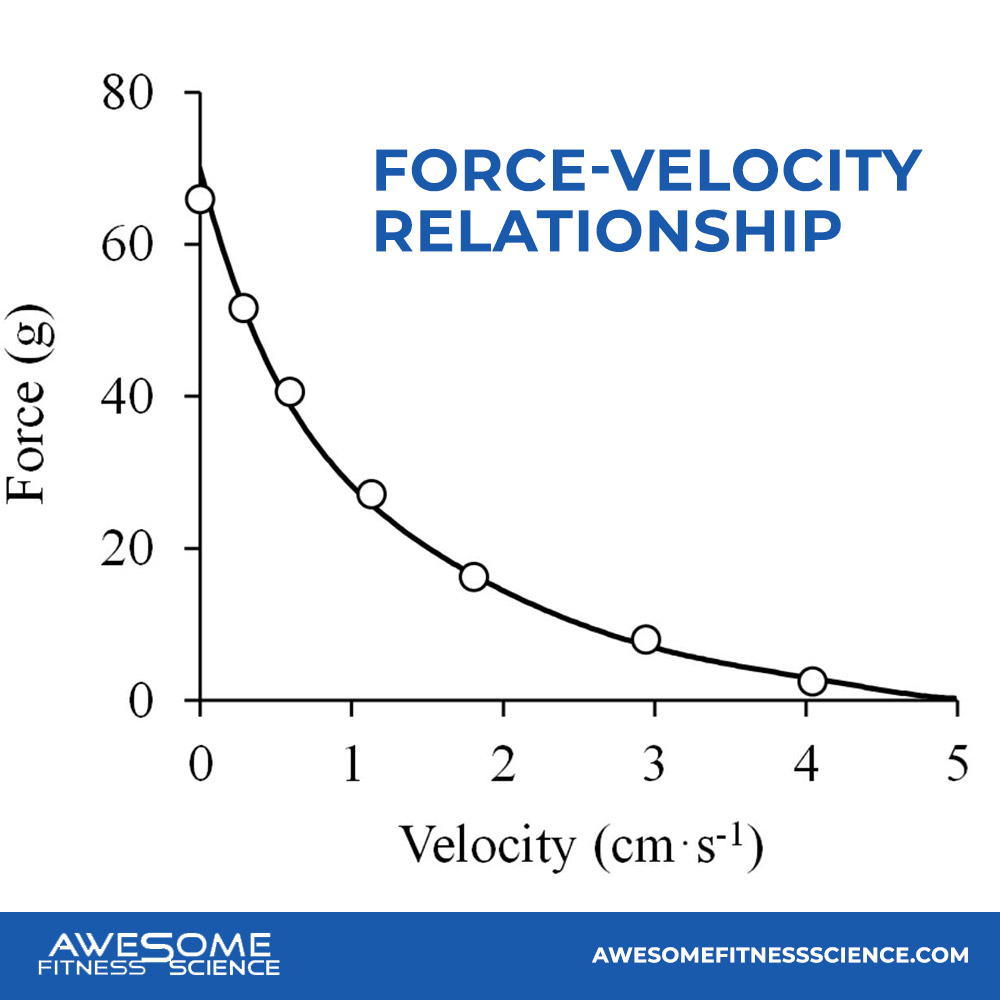
Furthermore, Newton’s Third Law also comes into play (11). There must be opposing force which slows down muscle contraction velocity. The opposing force attempts to stretch your muscle tissue while slowing down it’s contraction velocity. Thus, slow contraction velocities are crucial. If they weren’t, pie slapping would get you buff, but it doesn’t. While pie slapping achieves full motor unit recruitment, it doesn’t have a slow contraction velocity.
Here’s what’s happening at the deepest level. Keep reading because this will be critical to understand for a lifetime and is not just nerd stuff for professionals.
Remember how I mentioned actin and myosin are the units that contract your muscles when they unite. The more cross bridges actin and myosin form and the longer they overlap, the more force the individual fiber produces.
Essentially, you want as many actin and myosin sexual intercourses as possible and you want them to stay in intercourse as long as possible (13). With fast muscle contractions, actin and myosin don’t form as many cross bridges and they essentially stop mingling. They’re essentially having lackluster quickies as opposed to long mind-blowing sex.
On a side note, the length-tension relationship also impacts how much force is produced (16,17,18). The longer a muscle length, the more muscle force is produced. Think, when a rubber band is stretched. This is why the best muscle building exercises forces the muscles to shorten and stretch (also called the stretch-shortening cycle) as much in line with the full range of motion of the fibers.
I won’t go over which exercises accomplishes this best, but that will be for another future article.
So now, you know the prerequisite to muscle growth is for:
Mechanical tension is the sum of the 2 points above which also acts as the mechanical signal that needs to be translated. When sufficient mechanical tension is present, mechanotransduction occurs (19-23).
Mechanotransduction is the process by which your muscle cells/fibers adapt to the mechanical force occurring. It translates that tension nerdy stuff we just discussed into a cascade of chemical responses which eventually uses the DNA blueprint within your muscles to get it bigger by ordering for the construction of new muscle proteins. This final process is called muscle protein synthesis where your muscles grow in size. Cheers to that cause it makes our arms and butt look hot.
Trust me, most fitness professionals don’t know most of the above. Certifications (and even universities) don’t teach this stuff accurately or at all. So, you’re already smarter than many, but the education doesn’t stop. Time to learn how this applies in practice.
Load and fatigue work together to apply mechanical tension, assuming the exercise selection is good enough for hypertrophy.
Let’s start with load. Load is a proxy for how much external force is required per rep and how much resistance is applied per rep. The heavier the load, the more motor units are recruited and the slower the contraction velocity speed.
Think about it. A 200 lb bench press will require more force than a 100 lb bench press. Furthermore, benching 200 lbs will take longer per rep than benching 100 lbs. So if you lift heavy weights (like your 1-6 rep max), each rep imposes enough mechanical tension for muscle growth because full motor unit recruitment and a slow enough contraction velocity occurs immediately.
Spoiler alert. No. More weight does not mean more muscle growth. If you lift your 1-rep max to failure, you’d lift 1 rep. If you lift your 3-rep max to failure, you’d lift 3 reps.
The 1-rep max imposed more mechanical tension than each individual rep from your 3-rep max, but the 3-rep max accumulated a higher volume of mechanical tension. In fact, research indicates that lifting too heavy like 1-rep maxes is suboptimal for muscle growth on a per set basis because you don’t accumulate as much total tension.
With light weights, they don’t initially cause full motor unit recruitment or a slow contraction velocity. However, as I mentioned fatigue can also work together to apply mechanical tension. If you lift a light weight towards or up to failure, fatigue accumulates.
Fatigue does 2 things here:
Since both prerequisites for robust mechanical tension is present, light weights are extremely viable for hypertrophy. In fact, apart from extremely low reps or extremely high reps, research finds any reasonable rep range stimulates the same muscle growth by the end of the set assuming the same proximity to failure. There are essentially multiple paths to the same amount of mechanical tension.
With heavier weight/lower reps, full motor unit recruitment + slow contraction velocity occurs sooner in the set while you accumulate fewer yet heavier reps by the end of the set.
With lighter weights/heavier reps, the same full motor recruitment + slow contraction velocity occurs, but just later in the set while you accumulate more yet lighter reps by the end of the set.
The nuances are different, but the mechanical tension by the end of the set is equal and thus the muscle growth on a per set basis is also equal.

As fatigue slows down your concentric velocity, your reps become significantly more hypertrophic.
The opposite tempo can still cause hypertrophy, but is less effective. A deliberately slow concentric has a slow contraction velocity, but because it’s deliberately slow, total strength performance decreases via load and/or reps.
Furthermore, a fast eccentric uses gravity to lower the weight as opposed to the muscle producing it’s own force, so there is little to no mechanical tension for the lowering phase. In addition, if you lower weights too fast, you won’t be able to emphasize the stretched position of a rep which allows for both active and passive mechanical tension to occur. That concept is beyond the scope of this already extensive article, but I did talk about it in this article if you want to get into that rabbit hole.

But anyways, now you know what drives muscle growth. Mechanical tension does. In fact, that is the only stimulus that directly grows muscle despite what you might hear about other mechanisms. If you don’t believe me, allow me to dump a couple more thousand words about why the evidence is hysterically weak for other proposed mechanisms.
When oxygen is limited (hypoxia) within a muscle, the body transforms glucose into lactate. Your muscles begin to collect metabolic byproducts like lactate along phosphate and hydrogen ions (24). These by products are called metabolites which give you that burning pump feeling in your muscles also known as the muscle pump.
This is not to be confused with lactic acid which is different than the aforementioned metabolite lactate (25).
Metabolic stress has many proposed mechanisms for growing muscle, but they are all not supported strongly enough to conclude muscle growth (26,27). At best, all the proposed mechanisms are indirect mechanisms that rely on mechanical tension and at worst, some proposed mechanisms don’t do anything hypertrophy related.
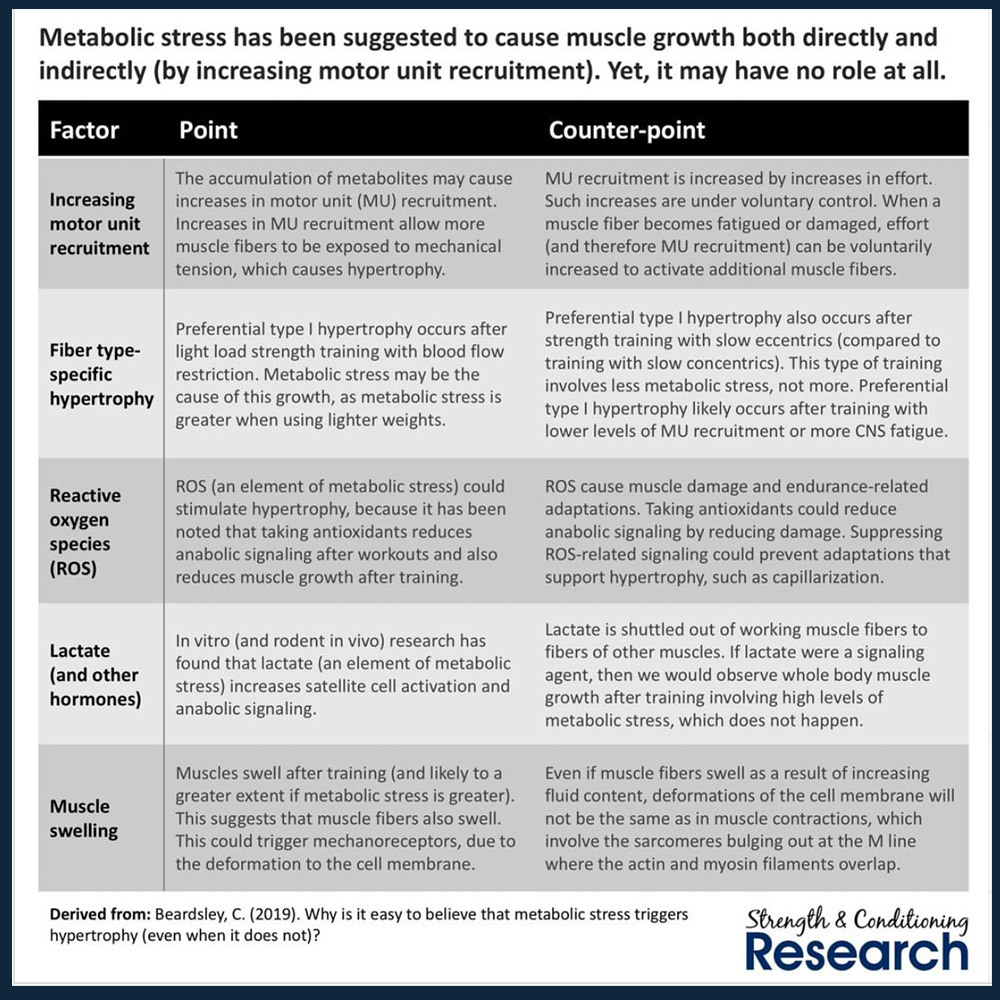
Metabolites simply don’t enhance muscle hypertrophy despite how good the pump sensation feels (28). Let’s sift through the evidence further:
Metabolic stress can increase muscle activation and allow for mechanical tension to occur by increasing fatigue and allowing for higher threshold motor units to be recruited with low weight.
However, metabolic stress itself is merely fluid accumulation between muscle cells causing them to swell. If you chase the pump, you may look bigger temporarily which has applications for contest prep and photoshoots, but the pump itself doesn’t guarantee lasting increases in muscle size.
Many people are taught that to build muscle, you have to break them down. You have to annihilate them, destroy them, or demolish them into submission, so they grow back bigger right? Wrong.
Building something back up and rising to new heights after being broken might make for some good motivational speaking, but is not an accurate model of hypertrophy.

First, let’s go over what muscle damage is.
When mechanical tension is inflicted on muscle fibers, damage will occur which isn’t surprising. Muscle fibers are long thin cylinders susceptible to micro tears especially when lengthened.
Disruption in a muscle fiber’s structure impacts the sarcomeres first. The outer membrane gets damaged later and is correlated with the degree of muscle stretch imposed. The mechanism behind all this is calcium build up within the muscle cell which strips the muscle fiber away of it’s structure (46). As a result, muscle contents can leak out of it’s wrapping layer into the bloodstream also detected as creatine kinase (47).
An acute inflammatory response occurs which signals for repair (48,49). White blood cells get attracted to help repair damaged fibers. Necrosis also occurs which essentially means some muscle fibers die out from damage and get regenerated (50).
The inflammatory response also signals for fluid and blood proteins to join in on the repair process which causes muscle swelling. This is why you can look more jacked after a couple days of working out despite 2 workouts building little actual muscle growth.
External load, total training volume, sustained muscle contractions, fatigue, novel muscle patterns, eccentric contractions, deep muscle stretch, compression, and laceration all contribute to causing muscle damage (51-55). But as you’ll see, the damage process itself doesn’t grow any muscle regardless of the rationale you try to justify.
Due to the inflammatory process of muscle damage, satellite cells are activated which leads some people to believe it causes more muscle growth, but this is untrue. In the words of Menno Henselmans, “Muscle hypertrophy can occur without satellite cell activation and satellite cell activation does not prove muscle growth is occurring.”
Research finds satellite cell activation is similar between strength training and cardio likely due to novelty, but you’d be a dimwit to believe cardio builds as much muscle (56).
Indeed, the more muscle damage you accumulate at the start of a training program due to novelty doesn’t translate to additional muscle growth even when satellite cell activation was higher (57,58,59).
Muscle damage also signals for higher levels of muscle protein synthesis which on surface sounds like it means more muscle. However, muscle damage is inherently muscle breakdown. You only get net muscle growth if muscle protein synthesis exceeds muscle breakdown.
So while the turnover of muscle proteins is higher with more muscle damage, the additional levels of muscle protein synthesis is used to repair the damage leaving no additional muscle growth (60,61).
In other words, muscle damage signals for muscle repair not muscle growth (80).
Eccentric contractions generally cause a bit more muscle growth given the same number of sets (62,63). Eccentric contractions also cause more muscle damage due to calcium buildup during muscle lengthening which leads people to conclude muscle damage is a mechanism of hypertrophy.
However, eccentrics only cause more muscle damage when you’re unaccustomed to them early on in a strength training program (64). Furthermore, any benefit from eccentric training can be explained with force production.
Your muscles are stronger during eccentric contractions (65,66). This is thanks to the third protein filament titin which is a passive structure contributing to force enhancement as a muscle lengthens (67,68,69).
This means, eccentric contractions are more hypertrophic because you can produce greater force levels, lift more total load, and do more total work. That sounds a lot like mechanical tension because wink, wink, it is. It’s not the muscle damage enhancing the growth, thus the credit goes back to mechanical tension (70,79).
If you still want to argue the model of muscle damage building muscle, I have more facts on facts to pimp slap you with.
People who grow more muscle don’t experience more muscle damage (71). In fact, one study compared one group who were new to a training program while one group had worked their way up to that program to minimize muscle damage (72). Despite one group experiencing far less muscle damage, both groups grew the same muscle within the program.
When you look at gender physiology, women experience less muscle damage yet they have the same muscle growth potential as men (relative to their starting point and body weight) (73).
One study went so far to isolate muscle damage and applied compressive loading aka extreme bruising on rats (75). Muscle damage levels skyrocketed, but if muscle damage is a driver of hypertrophy, we would see muscle growth, which didn’t happen. In fact, muscle was loss because of excessive damage which brings me to my next point.
Muscle damage can delay the process of muscle growth due to excessive repairs taking place (76). Think of a toxic relationship. How can it grow and progress if it’s constantly having to repair the deficits taking place?
Excess muscle damage also hinders recovery and motor pattern learning, both of which can cause overtraining and increase injury risk (77,78). This begs the question, how do you know how much muscle damage is present?
Most people would say soreness, but as you’ll see below, it’s not that straightforward. Let’s go over the mechanism of soreness. If you need a break, go take one because I’m likely about to dump at least another 1000 words on you cause of you know, passion.
Soreness can occur from a multitude of mechanisms via exercise. If soreness occurs, it usually appears the day after your workout and can peak in pain around 48 hours post workout (81). This is called delayed onset muscle soreness or DOMS for short.
As you might expect, muscle damage is related to soreness, but muscle damage is only one mechanism behind soreness. Soreness is also caused by connective tissue damage post training (85-88). Muscle fiber and connective tissue swelling also trigger sensory receptors in the skin (89). If that wasn’t nuanced enough, get this. Soreness rises and climbs it’s peaks after the workout, but muscle damage gets repaired and decreases after the workout, so the time course for their peaks and presence are different (82).
So soreness can indicate muscle damage has occurred, but soreness can’t indicate if muscle damage is still present, nor can soreness correlate with the level of muscle damage occurring (83,84). So you can have no soreness, but be insufficiently recovered. Conversely, you can be quite sore, but be fully recovered. Essentially, soreness is a confusing hoe.
Soreness is subjective in nature, so it’s a poor indicator of how well you’re recovered which is why I generally recommend for clients to still attempt to train even when they’re sore (assuming soreness is not excessive).
So all this to say, soreness barely correlates with muscle damage and definitely doesn’t cause hypertrophy either (90,91).
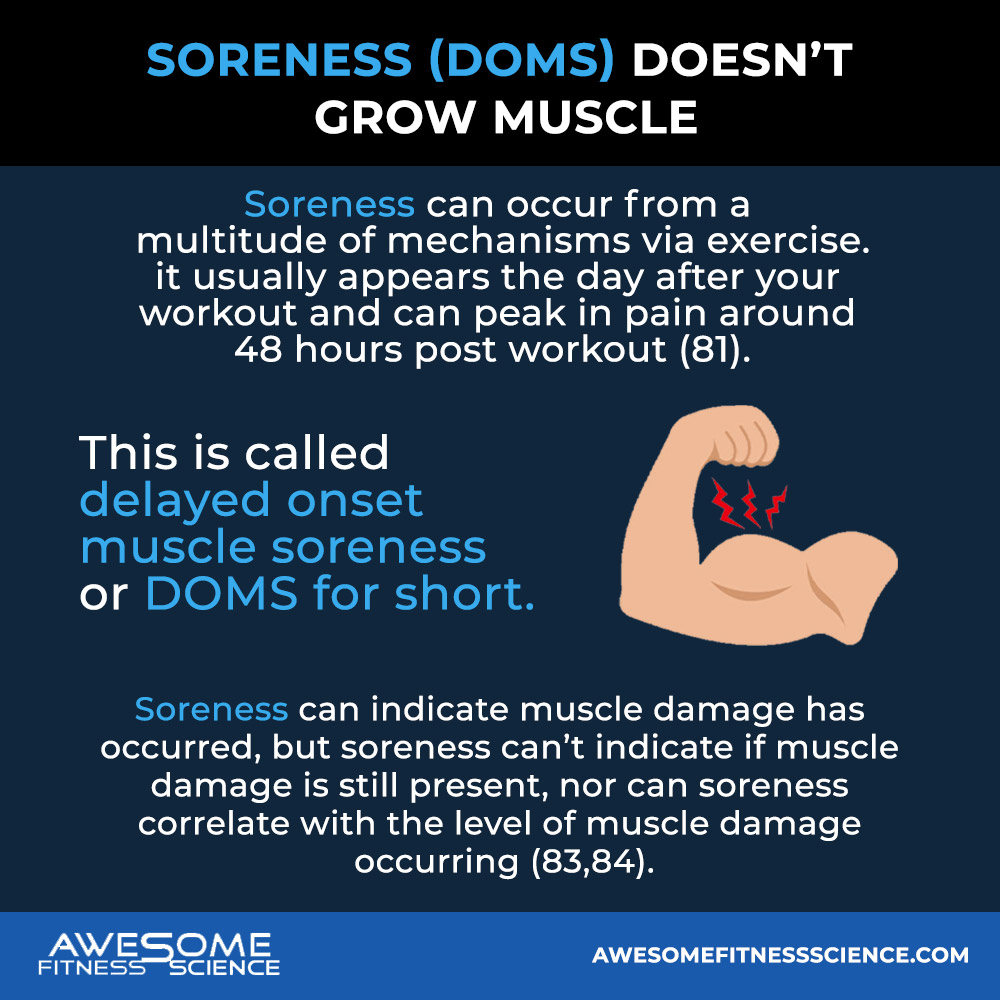
However, most people believe the opposite which is the sorer you are, the better the workout and more muscle you’ll grow. This can lead to bad or ineffective training decisions. For instance, you can easily chase soreness too much and lose muscle. Or you can stop an effective program because it stopped making you sore.
In fact, the repeated bout effect should naturally reduce average soreness you experience across a well-designed training program (92). The repeated bout effect basically states that the more you repeat an exercise, the less sore you’ll be from it even if you repeat it weeks later (93).
Your body starts to learn the neural patterns for that exercise and less muscle damage and DOMS occur (94). While soreness can decrease quite fast with early and frequent repeated bouts, some study finds it persists for up to 2 months (95-98).
Honestly, soreness doesn’t tell you much. It’s ultimately a feeling that hints at certain factors within your training, but it can’t dictate future training decisions. Some people would argue that some soreness could indicate that a muscle is sufficiently disrupted or that it at least let’s you know you’ve trained that muscle.
Here’s my gripe with these arguments.
So we’re back at the conclusion of, “Holy crap, soreness is a confusing hoe that I probably shouldn’t worry much about.” Yes, that’s what I’ve been trying to tell people for like ever. The only safe yet wildly obvious applications I can give for soreness are as follows:
The only direct mechanism of hypertrophy is mechanical tension. It is the driving force behind muscle growth. Anything else is either a byproduct or a distraction.
So stop chasing the pump, stop chasing damage, and stop wearing soreness as a badge of honor. You should be chasing performance. Figure out how you can get your body to lift heavier and do more total work over time given the mechanical tension model I’ve described above.
If you can’t do that, you’ll be working out endlessly with no muscle growth to show for despite getting gnarly pumps and feeling sore every other day.
1.
Archives.albion.edu.
https://archives.albion.edu/items/browse?sort_field___=&page=1&output=omeka-xml.
2.
Herzog, Walter, et al. “A New Paradigm for Muscle
Contraction.” Frontiers, Frontiers, 1 Jan. 1AD,
https://www.frontiersin.org/articles/10.3389/fphys.2015.00174/full.
3.
Purves, Dale. “Neuroscience.” National Center for
Biotechnology Information, U.S. National Library of Medicine, 1 Jan. 1970,
https://www.ncbi.nlm.nih.gov/books/NBK10799/.
4.
Potvin, Jim R., and Andrew J. Fuglevand. “A Motor
Unit-Based Model of Muscle Fatigue.” PLOS Computational Biology, Public
Library of Science, https://journals.plos.org/ploscompbiol/article?id=10.1371%2Fjournal.pcbi.1005581.
5.
J;, Miller. “Voluntary Activation and Central
Activation Failure in the Knee Extensors in Young Women and Men.” Scandinavian
Journal of Medicine & Science in Sports, U.S. National Library of
Medicine, https://pubmed.ncbi.nlm.nih.gov/16895533/.
6.
Wernbom, Mathias, and Per Aagaard. “Muscle Fibre
Activation and Fatigue with Low‐Load Blood Flow Restricted Resistance
Exercise-an Integrative Physiology Review.” Wiley Online Library, John
Wiley & Sons, Ltd, 18 June 2019,
https://onlinelibrary.wiley.com/doi/abs/10.1111/apha.13302.
7.
Gordon, Tessa, et al. “The Resilience of the Size
Principle in the Organization of Motor Unit Properties in Normal and
Reinnervated Adult Skeletal Muscles.” Canadian Journal of Physiology and
Pharmacology, 1 July 2004, https://cdnsciencepub.com/doi/10.1139/y04-081.
8.
Bawa, Parveen N S, et al. “Assessment of Size Ordered
Recruitment.” Frontiers in Human Neuroscience, Frontiers Media S.A., 28
July 2014, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4112781/.
9.
“Henneman’s Size Principle.” Wikipedia,
Wikimedia Foundation, 22 July 2020,
https://en.wikipedia.org/wiki/Henneman%27s_size_principle.
10. VF;,
Lenzen. “Newton’s Third Law.” Science (New York, N.Y.), U.S. National
Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/17753519/.
11. CL;,
Harwood. “Changes in Motor Unit Recruitment Thresholds of the Human Anconeus
Muscle during Torque Development Preceding Shortening Elbow Extensions.” Journal
of Neurophysiology, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/22378176/.
12. Piazzesi
. “Skeletal Muscle Performance Determined by Modulation of Number of Myosin
Motors Rather than Motor Force or Stroke Size.” Cell, U.S. National
Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/18022371/.
13. “Force
Velocity Relationship.” Force Velocity Relationship – an Overview |
ScienceDirect Topics,
https://www.sciencedirect.com/topics/engineering/force-velocity-relationship.
14. Alcazar,
Julian, et al. “On the Shape of the Force-Velocity Relationship in Skeletal
Muscles: The Linear, the Hyperbolic, and the Double-Hyperbolic.” Frontiers,
Frontiers, 1 Jan. 1AD,
https://www.frontiersin.org/articles/10.3389/fphys.2019.00769/full.
15. J;,
Brughelli. “Altering the Length-Tension Relationship with Eccentric Exercise :
Implications for Performance and Injury.” Sports Medicine (Auckland, N.Z.),
U.S. National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/17722950/.
16. Winters,
Taylor M, et al. “Whole Muscle Length-Tension Relationships Are Accurately
Modeled as Scaled Sarcomeres in Rabbit Hindlimb Muscles.” Journal of
Biomechanics, U.S. National Library of Medicine, 4 Jan. 2011,
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3003754/.
17. Visual
Attention – Home – Springer. https://link.springer.com/referenceworkentry/10.1007%2F978-3-540-29678-2_6344.
18. SM;,
West. “Human Exercise-Mediated Skeletal Muscle Hypertrophy Is an Intrinsic
Process.” The International Journal of Biochemistry & Cell Biology,
U.S. National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/20541030/.
19. Wong,
Victor W., et al. “Pushing Back: Wound Mechanotransduction in Repair and
Regeneration.” Journal of Investigative Dermatology, Elsevier, 8 Dec.
2015, https://www.sciencedirect.com/science/article/pii/S0022202X15350879.
20. Laplante,
Mathieu, and David M. Sabatini. “MTOR Signaling at a Glance.” Journal of
Cell Science, The Company of Biologists, 15 Oct. 2009,
https://journals.biologists.com/jcs/article/122/20/3589/30940/mTOR-signaling-at-a-glance.
21. “Mechanotransduction.”
Mechanotransduction – an Overview | ScienceDirect Topics,
https://www.sciencedirect.com/topics/medicine-and-dentistry/mechanotransduction.
22. Burkholder,
Thomas J. “Mechanotransduction in Skeletal Muscle.” Frontiers in Bioscience
: a Journal and Virtual Library, U.S. National Library of Medicine, 1 Jan.
2007, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2043154/.
23. BJ;,
Schoenfeld. “Potential Mechanisms for a Role of Metabolic Stress in
Hypertrophic Adaptations to Resistance Training.” Sports Medicine (Auckland,
N.Z.), U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/23338987/.
24. SP;,
Cairns. “Lactic Acid and Exercise Performance : Culprit or Friend?” Sports
Medicine (Auckland, N.Z.), U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/16573355/.
25. de
Freitas, Marcelo Conrado, et al. “Role of Metabolic Stress for Enhancing Muscle
Adaptations: Practical Applications.” World Journal of Methodology,
Baishideng Publishing Group Inc, 26 June 2017,
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5489423/.
26. Beardsley,
Chris. “Why Is It Easy to Believe That Metabolic Stress Triggers Hypertrophy
(Even When It Does Not)?” Medium, Medium, 18 Apr. 2021,
https://sandcresearch.medium.com/why-is-it-easy-to-believe-that-metabolic-stress-triggers-hypertrophy-even-when-it-does-not-2928928a431.
27. Dankel
. “Do Metabolites That Are Produced during Resistance Exercise Enhance Muscle
Hypertrophy?” European Journal of Applied Physiology, U.S. National
Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/28776271/.
28. P;,
Cipryan. “Acute and Post-Exercise Physiological Responses to High-Intensity
Interval Training in Endurance and Sprint Athletes.” Journal of Sports
Science & Medicine, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/28630575/.
29. Fink,
Julius, et al. “Effects of Rest Intervals and Training Loads on Metabolic
Stress and Muscle Hypertrophy.” Wiley Online Library, John Wiley &
Sons, Ltd, 28 Dec. 2016,
https://onlinelibrary.wiley.com/doi/abs/10.1111/cpf.12409.
30. SM;,
de Morree. “Perception of Effort Reflects Central Motor Command during Movement
Execution.” Psychophysiology, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/22725828/.
31. K;,
Nielsen. “Effects of Lengthening Contraction on Calcium Kinetics and Skeletal
Muscle Contractility in Humans.” Acta Physiologica Scandinavica, U.S.
National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/15954988/.
32. Farup
. “Blood Flow Restricted and Traditional Resistance Training Performed to
Fatigue Produce Equal Muscle Hypertrophy.” Scandinavian Journal of Medicine
& Science in Sports, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/25603897/.
33. Health,
Department of. “The Use of Occlusion Training to Produce Muscle Hypertrophy :
Strength & Conditioning Journal.” LWW,
https://journals.lww.com/nsca-scj/FullText/2009/06000/The_Use_of_Occlusion_Training_to_Produce_Muscle.11.aspx.
34. Nyakayiru,
Jean, et al. “Blood Flow Restriction Only Increases Myofibrillar Protein
Synthesis with Exercise.” Medicine and Science in Sports and Exercise,
Lippincott Williams & Wilkins, June 2019,
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6553970/.
35. “The
Efficacy of Resistance Training in Hypoxia to Enhance Strength and Muscle
Growth: A Systematic Review and Meta-Analysis.” Taylor & Francis,
https://www.tandfonline.com/doi/abs/10.1080/17461391.2017.1388850?journalCode=tejs20.
36. Liegnell
. “Elevated Plasma Lactate Levels via Exogenous Lactate Infusion Do Not Alter
Resistance Exercise-Induced Signaling or Protein Synthesis in Human Skeletal
Muscle.” American Journal of Physiology. Endocrinology and Metabolism,
U.S. National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/32830552/.
37. Teixeira
. “Blood Flow Restriction Does Not Attenuate Short-Term Detraining-Induced
Muscle Size and Strength Losses after Resistance Training with Blood Flow
Restriction.” Journal of Strength and Conditioning Research, U.S.
National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/31009425/.
38. Lixandrão.
“Effects of Exercise Intensity and Occlusion Pressure after 12 Weeks of
Resistance Training with Blood-Flow Restriction.” European Journal of
Applied Physiology, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/26323350/.
39. MacDougall
. “Arterial Blood Pressure Response to Heavy Resistance Exercise.” Journal
of Applied Physiology (Bethesda, Md. : 1985), U.S. National Library of
Medicine, https://pubmed.ncbi.nlm.nih.gov/3980383/.
40. Blood
Ammonia and Lactate as Markers of Muscle Metabolites …
https://www.researchgate.net/publication/261743054_Blood_Ammonia_and_Lactate_as_Markers_of_Muscle_Metabolites_During_Leg_Press_Exercise.
41. Pareja-Blanco.
“Time Course of Recovery from Resistance Exercise with Different Set
Configurations.” Journal of Strength and Conditioning Research, U.S.
National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/30036284/.
42. (PDF)
Resistance Training with Slow Speed of Movement Is …
https://www.researchgate.net/publication/305676699_Resistance_training_with_slow_speed_of_movement_is_better_for_hypertrophy_and_muscle_strength_gains_than_fast_speed_of_movement.
43. Goto,
Kazushige, et al. “Hormonal and Metabolic Responses to Slow Movement Resistance
Exercise with Different Durations of Concentric and Eccentric Actions.” European
Journal of Applied Physiology, Springer-Verlag, 10 May 2009,
https://link.springer.com/article/10.1007%2Fs00421-009-1075-9.
44. JW;,
Schoenfeld. “Effect of Repetition Duration during Resistance Training on Muscle
Hypertrophy: A Systematic Review and Meta-Analysis.” Sports Medicine
(Auckland, N.Z.), U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/25601394/.
45. Kano,
Yutaka, et al. “Mechanisms of Exercise-Induced Muscle Damage and Fatigue:
Intracellular Calcium Accumulation.” The Journal of Physical Fitness and
Sports Medicine, The Japanese Society of Physical Fitness and Sports
Medicine, 23 Oct. 2012, https://www.jstage.jst.go.jp/article/jpfsm/1/3/1_505/_article.
46. “(PDF)
Creatine Kinase Monitoring in Sport Medicine.” ResearchGate,
https://www.researchgate.net/publication/6266891_Creatine_Kinase_Monitoring_In_Sport_Medicine.
47. BJ;,
Schoenfeld. “The Mechanisms of Muscle Hypertrophy and Their Application to
Resistance Training.” Journal of Strength and Conditioning Research,
U.S. National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/20847704/.
48. FX;,
Koh. “Do Inflammatory Cells Influence Skeletal Muscle Hypertrophy?” Frontiers
in Bioscience (Elite Edition), U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/19482625/.
49. Hyldahl,
Robert D., and Monica J. Hubal. “Lengthening Our Perspective: Morphological,
Cellular, and Molecular Responses to Eccentric Exercise.” Wiley Online
Library, John Wiley & Sons, Ltd, 3 Dec. 2013,
https://onlinelibrary.wiley.com/doi/10.1002/mus.24077.
50. TA;,
Butterfield. “Eccentric Exercise in Vivo: Strain-Induced Muscle Damage and
Adaptation in a Stable System.” Exercise and Sport Sciences Reviews,
U.S. National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/20335736/.
51. Gibala
. “Myofibrillar Disruption Following Acute Concentric and Eccentric Resistance
Exercise in Strength-Trained Men.” Canadian Journal of Physiology and
Pharmacology, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/10958167/.
52. Wernbom.
“Contractile Function and Sarcolemmal Permeability after Acute Low-Load
Resistance Exercise with Blood Flow Restriction.” European Journal of
Applied Physiology, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/21947453/.
53. WE;,
Mair. “The Role of Fatigue in Susceptibility to Acute Muscle Strain Injury.” The
American Journal of Sports Medicine, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/8775109/.
54. Brooks,
S V, et al. “Injury to Muscle Fibres after Single Stretches of Passive and
Maximally Stimulated Muscles in Mice.” The Journal of Physiology, U.S.
National Library of Medicine, 15 Oct. 1995,
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1156684/.
55. Verney
. “Effects of Combined Lower Body Endurance and Upper Body Resistance Training
on the Satellite Cell Pool in Elderly Subjects.” Muscle & Nerve,
U.S. National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/18671293/.
56. Damas
. “Early- and Later-Phases Satellite Cell Responses and Myonuclear Content with
Resistance Training in Young Men.” PloS One, U.S. National Library of
Medicine, https://pubmed.ncbi.nlm.nih.gov/29324825/.
57. Hyldahl
RD;Nelson B;Xin L;Welling T;Groscost L;Hubal MJ;Chipkin S;Clarkson PM;Parcell
AC; “Extracellular Matrix Remodeling and Its Contribution to Protective
Adaptation Following Lengthening Contractions in Human Muscle.” FASEB
Journal : Official Publication of the Federation of American Societies for
Experimental Biology, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/25808538/.
58. Damas
. “Resistance Training-Induced Changes in Integrated Myofibrillar Protein
Synthesis Are Related to Hypertrophy Only after Attenuation of Muscle Damage.” The
Journal of Physiology, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/27219125/.
59. C;,
Damas. “The Development of Skeletal Muscle Hypertrophy through Resistance
Training: The Role of Muscle Damage and Muscle Protein Synthesis.” European
Journal of Applied Physiology, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/29282529/.
60. Roig
. “The Effects of Eccentric versus Concentric Resistance Training on Muscle
Strength and Mass in Healthy Adults: A Systematic Review with Meta-Analysis.” British
Journal of Sports Medicine, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/18981046/.
61. Schoenfeld.
“Hypertrophic Effects of Concentric vs. Eccentric Muscle Actions: A Systematic
Review and Meta-Analysis.” Journal of Strength and Conditioning Research,
U.S. National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/28486337/.
62. Margaritelis
. “Eccentric Exercise per Se Does Not Affect Muscle Damage Biomarkers: Early
and Late Phase Adaptations.” European Journal of Applied Physiology,
U.S. National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/33156414/.
63. 1Department
of Kinesiology and Health Studies. “Maximal Eccentric and Concentric Strength
Discrepancies… : The Journal of Strength & Conditioning Research.” LWW,
https://journals.lww.com/nsca-jscr/Abstract/2007/02000/MAXIMAL_ECCENTRIC_AND_CONCENTRIC_STRENGTH.7.aspx.
64. Kinesiology,
1Department of. “Comparison of Concentric and Eccentric Bench Press… : The
Journal of Strength & Conditioning Research.” LWW,
https://journals.lww.com/nsca-jscr/Fulltext/2015/04000/Comparison_of_Concentric_and_Eccentric_Bench_Press.23.aspx.
65. Herzog,
Walter. “Why Are Muscles Strong, and Why Do They Require Little Energy in
Eccentric Action?” Journal of Sport and Health Science, Elsevier, 2 June
2018, https://www.sciencedirect.com/science/article/pii/S2095254618300462.
66. “Home
– PMC – NCBI.” National Center for Biotechnology Information, U.S.
National Library of Medicine, https://www.ncbi.nlm.nih.gov/pmc/.
67. Ashida.
“Effects of Contraction Mode and Stimulation Frequency on Electrical
Stimulation-Induced Skeletal Muscle Hypertrophy.” Journal of Applied
Physiology (Bethesda, Md. : 1985), U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/29074713/.
68. Centre
for Exercise Science and Sports Management. “A Cross-Sectional Comparison of
Different Resistance… : The Journal of Strength & Conditioning Research.”
LWW,
https://journals.lww.com/nsca-jscr/abstract/1999/08000/a_cross_sectional_comparison_of_different.12.aspx.
69. Haun,
Cody T, et al. “Pre-Training Skeletal Muscle Fiber Size and Predominant Fiber
Type Best Predict Hypertrophic Responses to 6 Weeks of Resistance Training in
Previously Trained Young Men.” Frontiers in Physiology, Frontiers Media
S.A., 26 Mar. 2019, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6445136/.
70. Flann.
“Muscle Damage and Muscle Remodeling: No Pain, No Gain?” The Journal of
Experimental Biology, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/21270317/.
71. D;,
Kerksick. “Gender-Related Differences in Muscle Injury, Oxidative Stress, and
Apoptosis.” Medicine and Science in Sports and Exercise, U.S. National
Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/18799987/.
72. Morán-Navarro
. “Time Course of Recovery Following Resistance Training Leading or Not to
Failure.” European Journal of Applied Physiology, U.S. National Library
of Medicine, https://pubmed.ncbi.nlm.nih.gov/28965198/.
73. TF;,
Minamoto. “Regenerated Rat Skeletal Muscle after Periodic Contusions.” Brazilian
Journal of Medical and Biological Research = Revista Brasileira De Pesquisas
Medicas e Biologicas, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/11668355/.
74. Bjørnsen,
Thomas, et al. “Delayed Myonuclear Addition, Myofiber Hypertrophy, and
Increases in Strength with High-Frequency Low-Load Blood Flow Restricted
Training to Volitional Failure.” Journal of Applied Physiology, 15 Mar.
2019, https://journals.physiology.org/doi/full/10.1152/japplphysiol.00397.2018.
75. Macgregor,
Lewis J, and Angus M Hunter. “High-Threshold Motor Unit Firing Reflects Force
Recovery Following a Bout of Damaging Eccentric Exercise.” PloS One,
Public Library of Science, 9 Apr. 2018,
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5890972/.
76. Leite
. “Does Exercise-Induced Muscle Damage Impair Subsequent Motor Skill Learning?”
Human Movement Science, U.S. National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/31362262/.
77. A;,
Fukada. “Role of Damage and Management in Muscle Hypertrophy: Different
Behaviors of Muscle Stem Cells in Regeneration and Hypertrophy.” Biochimica
Et Biophysica Acta. Molecular Cell Research, U.S. National Library of
Medicine, https://pubmed.ncbi.nlm.nih.gov/32417255/.
78. L;,
Cheung. “Delayed Onset Muscle Soreness : Treatment Strategies and Performance
Factors.” Sports Medicine (Auckland, N.Z.), U.S. National Library of
Medicine, https://pubmed.ncbi.nlm.nih.gov/12617692/.
79. Yu,
Ji-Guo, et al. “Evidence for Myofibril Remodeling as Opposed to Myofibril
Damage in Human Muscles with Doms: An Ultrastructural and Immunoelectron
Microscopic Study.” Histochemistry and Cell Biology, Springer-Verlag, 26
Feb. 2004, https://link.springer.com/article/10.1007/s00418-004-0625-9.
80. Nosaka,
Kazunori, et al. “Delayed‐Onset Muscle Soreness Does Not Reflect the Magnitude
of Eccentric Exercise‐Induced Muscle Damage.” Wiley Online Library, John
Wiley & Sons, Ltd, 9 Dec. 2002, https://onlinelibrary.wiley.com/doi/abs/10.1034/j.1600-0838.2002.10178.x.
81. Chen.
“Damage and the Repeated Bout Effect of Arm, Leg, and Trunk Muscles Induced by
Eccentric Resistance Exercises.” Scandinavian Journal of Medicine &
Science in Sports, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/30663816/.
82. Geisler,
P R, et al. “Changes in Tissue Degradation Markers and Subjective Reports of
Pain Resulting from Eccentric Muscle Contractions.” Biology of Sport,
U.S. National Library of Medicine, 1996, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7034258/.
83. Crameri,
R M, et al. “Myofibre Damage in Human Skeletal Muscle: Effects of Electrical
Stimulation versus Voluntary Contraction.” The Journal of Physiology,
Blackwell Science Inc, 15 Aug. 2007, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2277245/.
84. WM;,
Abraham. “Factors in Delayed Muscle Soreness.” Medicine and Science in
Sports, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/870780/.
85. AL;,
Mattiello-Sverzut. “Morphological Adaptation of Muscle Collagen and Receptor of
Advanced Glycation End Product (RAGE) in Osteoarthritis Patients with 12 Weeks
of Resistance Training: Influence of Anti-Inflammatory or Glucosamine
Treatment.” Rheumatology International, U.S. National Library of
Medicine, https://pubmed.ncbi.nlm.nih.gov/23443332/.
86. MJ;,
Lumpkin. “Mechanisms of Sensory Transduction in the Skin.” Nature, U.S.
National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/17314972/.
87. FL;,
Gomes. “High-Frequency Resistance Training Is Not More Effective than
Low-Frequency Resistance Training in Increasing Muscle Mass and Strength in
Well-Trained Men.” Journal of Strength and Conditioning Research, U.S.
National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/29489727/.
88. Science,
1Department of Health. “Is Postexercise Muscle Soreness a Valid Indicator of…
: Strength & Conditioning Journal.” LWW,
https://journals.lww.com/nsca-scj/Fulltext/2013/10000/Is_Postexercise_Muscle_Soreness_a_Valid_Indicator.2.aspx.
89. Chen
. “Damage and the Repeated Bout Effect of Arm, Leg, and Trunk Muscles Induced
by Eccentric Resistance Exercises.” Scandinavian Journal of Medicine &
Science in Sports, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/30663816/.
90. P;,
Hosseinzadeh. “Pain Sensitivity Is Normalized after a Repeated Bout of
Eccentric Exercise.” European Journal of Applied Physiology, U.S.
National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/23922170/.
91. Meneghel,
Adilson J., et al. “Review of the Repeated Bout Effect in Trained and Untrained
Men.” International Journal of Sports Science, Scientific & Academic
Publishing, http://article.sapub.org/10.5923.j.sports.20130305.02.html.
92. JM;,
Nosaka. “Time Course of Muscle Adaptation after High Force Eccentric Exercise.”
European Journal of Applied Physiology and Occupational Physiology, U.S.
National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/1915336/.
93. Dipasquale,
Dana M, et al. “Determinants of the Repeated-Bout Effect after Lengthening
Contractions.” American Journal of Physical Medicine & Rehabilitation,
U.S. National Library of Medicine, Oct. 2011,
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3169747/.
94. Byrnes
. “Delayed Onset Muscle Soreness Following Repeated Bouts of Downhill Running.”
Journal of Applied Physiology (Bethesda, Md. : 1985), U.S. National
Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/4055561/.
95. P;,
Nosaka. “How Long Does the Protective Effect on Eccentric Exercise-Induced
Muscle Damage Last?” Medicine and Science in Sports and Exercise, U.S.
National Library of Medicine, https://pubmed.ncbi.nlm.nih.gov/11528337/.
96. S;,
DiCarlo. “Experiment Demonstrating Skeletal Muscle Biomechanics.” The
American Journal of Physiology, U.S. National Library of Medicine,
https://pubmed.ncbi.nlm.nih.gov/16161227/.
97. Potvin,
Jim R., and Andrew J. Fuglevand. “A Motor Unit-Based Model of Muscle Fatigue.” PLOS
Computational Biology, Public Library of Science, https://journals.plos.org/ploscompbiol/article?id=10.1371%2Fjournal.pcbi.1005581.
98. Yu,
Ji-Guo, et al. “Evidence for Myofibril Remodeling as Opposed to Myofibril
Damage in Human Muscles with Doms: An Ultrastructural and Immunoelectron
Microscopic Study.” Histochemistry and Cell Biology, Springer-Verlag, 26
Feb. 2004, https://link.springer.com/article/10.1007/s00418-004-0625-9.
Grab my free Stupid Simple Scroll to Mastering Hypertrophy

What if I told you everything you knew about sugar is wrong? Like not just a little wrong, but so wrong, you might even get a brain scan after realizing all the lies you believed about sugar.

The forbidden fruit effect is the idea of restricting certain foods making you crave them more and thus, ruining your diet adherence or progress.

Think of your most stubborn muscle group that’s so painfully small despite how hard you train it. Perhaps, it’s your narrow back, your chopstick calves, or your flat butt.